Student Spotlight: Nanotechnology teaches trial, error and victory
/By Alex Herazy, senior at Wheeling High School
ALEX HERAZY AND PAUL SZCZEPANSKI
When Paul Szczepanski and I decided to team up to develop a project for our nanotechnology research project at Wheeling High School, our main goal was to create something that would positively impact humanity. In researching various ideas, we eventually decided to try to create a more effective medical bandage after our teacher directed us to a bucket of nanocellulose in the back of the Nanotechnology Lab.
Nanocellulose is an organic plant-based substance in a gel-like form. Its structure can best be compared to spaghetti: it’s composed of nano-sized spaghetti-like fibers that intertwine with each other. Preliminary research showed us that dry nanocellulose could absorb liquids in high amounts, so we thought this would be a perfect product for replacing current medical bandages.
To start, we spent a great deal of time on preliminary experiments trying to find the best way to dehydrate the gel-like nanocellulose. While many of our classmates were designing experiments based on primary research they had read, we couldn’t find any research that fit our ideas and had to generate our experiment from scratch. So we tried a lot of different methods of air drying, including using a dehydrator and an oven, with the virtual help of our mentor, Will Grubbe, who is working toward a Ph.D. in molecular engineering at the University of Chicago. He guided us through many trials and errors, suggesting possible temperature changes and other ideas after we “artistically reshaped” (melted) a few petri dishes.
Nanocellulose dried three ways, from left to right: 1) in an oven; 2) with a dehydrator for 20 minutes; and, 3) air-dried for a week
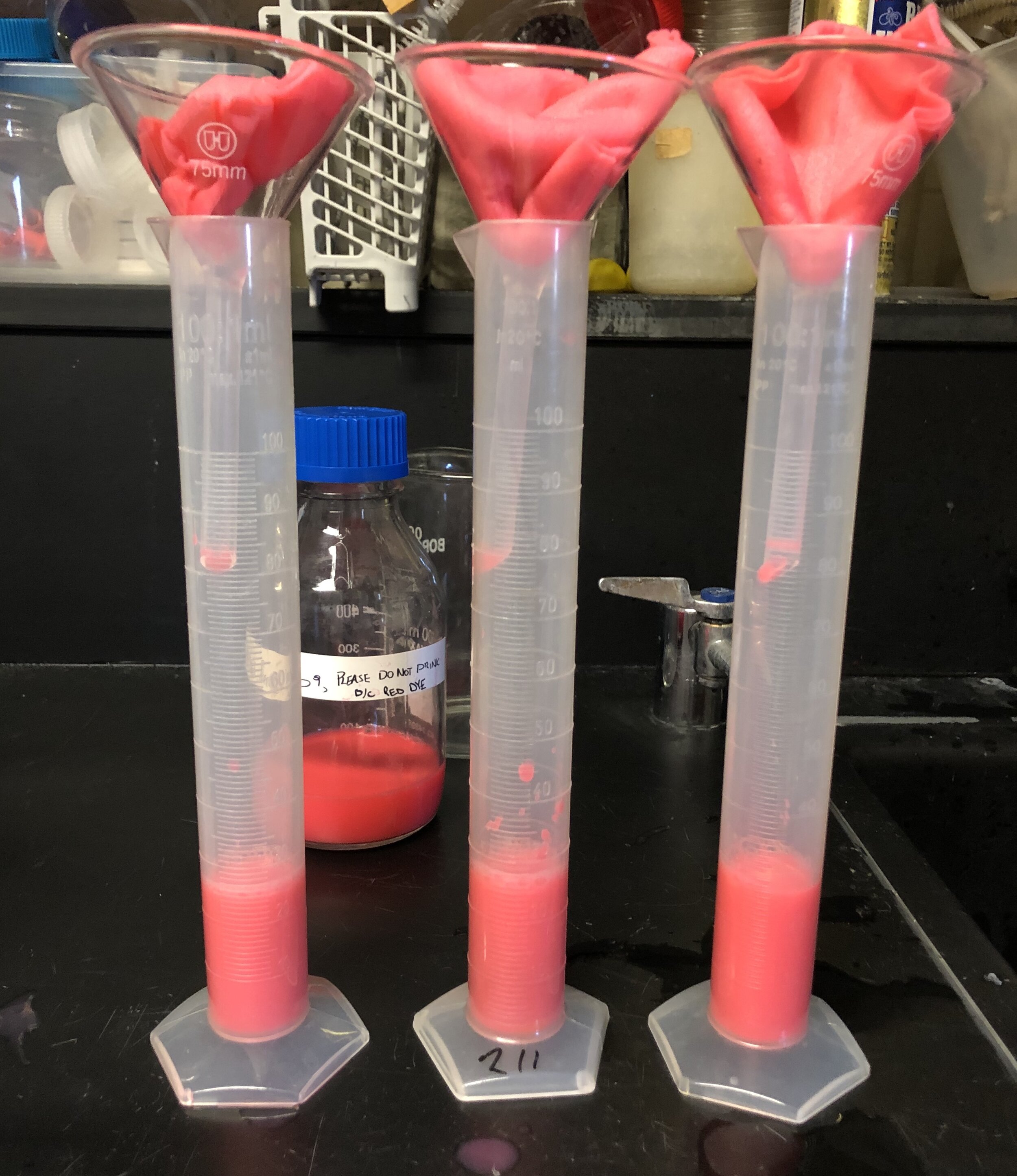
Our final experimental procedure involved changing the amount of dehydration time as determined by our preliminary experiments, then measuring the absorption of water and a synthetic blood with the nanocellulose samples we created. We also sprayed isopropyl alcohol on selected samples to add another variable that we thought might alter the nanocellulose porosity of our bandage and increase absorption. We completed three trials of this experiment and found nanocellulose at all dehydrated intervals was more effective at absorbing liquids than industry gauze.
This is what hydrated cellulose looks like
In all, we put more than 260 hours into this project and tried over 50 combinations of variables to create the most absorbent bandage. And it paid off.
Our project won a Gold Award at the Illinois Junior Academy of Science Regional Fair and was selected for the Illinois State Science Fair, which was later canceled due to the coronavirus pandemic. It also was honored as one of only 15 projects statewide to be selected for the Illinois Science & Technology Institute’s Student Research Showcase at the Microsoft Technology Center in Chicago, which was moved to a virtual Facebook showcase.
Industry gauze being tested with artificial blood we created with powdered milk, water and food dye to replicate the viscosity of blood
Paul and I learned a lot from this project, especially thanks to ISTE’s Mentor Matching Engine, an invitation-based web platform that connects Illinois high school students and their teachers to STEM professionals who serve as online mentors. That’s how we met Will Grubbe, who mentored us throughout the project. We also benefited from the guidance and support of our teacher, Carol Bouvier, who also discouraged us from using our own blood in our experiment but allowed us to wear lab coats to ensure we looked professional; a phenomenal substitute teacher, John Astreides, who stepped in for Ms. Bouvier when she was out for several weeks; Greg Wallace, a former University of Chicago researcher who currently teaches AP biology at Wheeling High School, who carefully reviewed and edited our 38-page project document; Bruce French, WHS division head for Math & Science, who helped with initial guidance and project planning; and Steven Bradley, a material scientist and president of Bradley Consulting Services who volunteered to work with WHS’s nanotechnology students and was always willing to offer advice.
Through this process we learned that science involves a great deal of trials and many errors. We also learned that one experiment can lead to others, and there are many possible ways to further this research. One idea would be to freeze-dry the nanocellulose to create a more porous surface when thawed. Additional research would include spraying the nanocellulose with other alcohols or even stirring the alcohol directly into the nanocellulose. Once the most effective treatment for nanocellulose absorption is developed, we would next research how to apply these results and create an effective medical bandage. But that will have to wait. Paul graduated in May and will attend DePaul University this fall, and I’m about to enter my senior year before going to college to study economics and public policy.